Neurons in Action in Action
Educational settings for simulations and tutorials using NEURON
Department of Cell & Molecular Physiology, 111 Mason Farm Rd, UNC, Chapel Hill, NC, 29599-7545, Phone: 919-966-0272, Fax: 919-966-6927, email: stuart@med.unc.eduurn:nbn:de:0009-3-14016
Abstract. Neurons in Action (NIA1, 2000; NIA1.5, 2004; NIA2, 2007), a set of tutorials and linked simulations, is designed to acquaint students with neuronal physiology through interactive, virtual laboratory experiments. Here we explore the uses of NIA in lecture, both interactive and didactic, as well as in the undergraduate laboratory, in the graduate seminar course, and as an examination tool through homework and problem set assignments. NIA, made with the simulator NEURON ( http://www.neuron.yale.edu/neuron/ ), displays voltages, currents, and conductances in a membrane patch or signals moving within the dendrites, soma and/or axon of a neuron. Customized simulations start with the plain lipid bilayer and progress through equilibrium potentials; currents through single Na and K channels; Na and Ca action potentials; voltage clamp of a patch or a whole neuron; voltage spread and propagation in axons, motoneurons and nerve terminals; synaptic excitation and inhibition; and advanced topics such as channel kinetics and coincidence detection. The user asks and answers "what if" questions by specifying neuronal parameters, ion concentrations, and temperature, and the experimental results are then plotted as conductances, currents, and voltage changes. Such exercises provide immediate confirmation or refutation of the student's ideas to guide their learning. The tutorials are hyperlinked to explanatory information and to original research papers. Although the NIA tutorials were designed as a sequence to empower a student with a working knowledge of fundamental neuronal principles, we find that faculty are using the individual tutorials in a variety of educational situations, some of which are described here. Here we offer ideas to colleagues using interactive software, whether NIA or another tool, for educating students of differing backgrounds in the subject of neurophysiology.
Keywords: Tutorial, simulation, action potenial, synaptic potential, ion channel, ion channel kinetics
The subject of neurophysiology can be intimidating to students who may fear electrical circuitry and cannot easily relate concepts such as conductance and capacitance to their general understanding of biology and physiology. Yet, with the subject of neuroscience expanding in so many directions, it seems essential that the neurobiologist have a grasp of the fundamental principles of neuronal function and an appreciation of how the field can be aided by computational tools. To this end, we (John W. Moore and I) authored a set of tutorials with interactive simulations on a CD, Neurons in Action (NIA) ( Moore and Stuart, 2000 ; 2007 ). Here, we detail the various ways in which this software has been used by us and by others. We offer these ideas to colleagues using interactive software, whether NIA or another tool, in hopes of increasing students' understanding of neurophysiology.
NIA exploits the power of its browser to run customized simulations using the simulator NEURON ( Hines and Carnevale 1997 , 2001 ; Carnevale and Hines 2006 ) under the direction of tutorials written in HTML. In 2000 we published NIA Version 1 (NIA1; Moore and Stuart 2000 ). We hoped that being able to ask "what if" questions, to change parameters, and then receive instant feedback from the simulations would make neurophysiology more approachable, even engaging for undergraduate and graduate students and fun and instructive even for the seasoned neuroscientist. In response to requests from colleagues, we later added a set of "minimovies" to this software ( Moore and Stuart 2004 ) for faculty who did not have the time available in their course to explore action potentials or synaptic potentials beyond one or two lectures. The minimovies illustrate fundamental aspects of neuronal and synaptic function.
In 2007, working with colleagues who use the program, we redesigned the interface and navigation, added new tutorials and revised others, linked the minimovie voltages to sound, and published these changes as NIA Version 2 (NIA2; Fig 1)( Moore and Stuart 2007 ). This effort was generously supported by the USA National Science Foundation. In all versions we link the tutorial text to explanations, answers to questions, historical context, and background material to aid both students and faculty in the interpretation of the simulations.

Figure 1: The Home Page of NIA2. Selecting Background allows the user then to choose History, an extensive exposition of the events and experiments culminating in the elucidation of the action potential, or PDFs, a selection of classic papers on the CD, including the Hodgkin-Huxley papers. Selecting Minimovies takes the user to the three categories of 19 minimovies detailed below. Selecting Tutorials brings up the list shown in Figure 2.
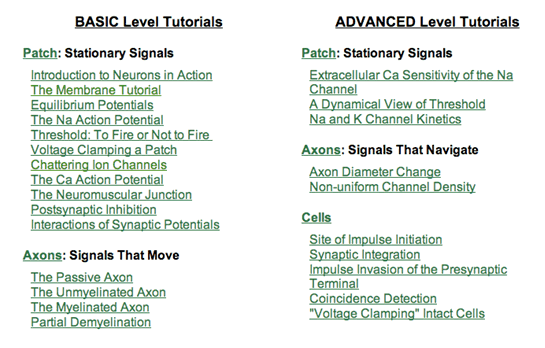
Figure 2: The set of 25 tutorials in NIA2. Tutorials are grouped into Basic and Advanced categories; within each category they are grouped by geometry.
Many faculty teaching in psychology, physiology, and medical school courses find that they have only one lecture in which to convey to students the nature of that miniature, propagating electrical explosion - the action potential. Typically a second lecture is devoted to synaptic transmission. Then the course material moves quickly on from these rather difficult topics to subjects such as memory or aggression, to which a student can more quickly relate. Faced with the problem of making the action and synaptic potentials more exciting, so to speak, we designed NIA minimovies for our colleagues to introduce into their PowerPoint lectures. In NIA2 we have attempted to make the minimovies still more lively by linking the voltage to sound.
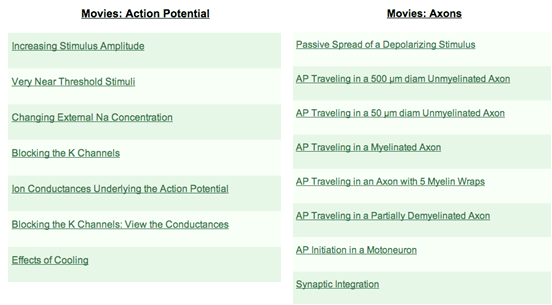
Figure 3. Lists of minimovies in the Action Potential and Axons categories. Minimovies of aspects of excitatory and inhibitory transmission are also available. The instructor can preview each minimovie before importing it into PowerPoint.
The minimovies (available since NIA1.5) are Quicktime movie files captured by the authors from NIA simulations. They are grouped into three categories: (1) the basic properties of the action potential in a patch, (2) the action potential as it propagates along unmyelinated, myelinated, or partially demyelinated nerve (Fig 3), and (3) the salient features of excitatory and inhibitory postsynaptic potentials. Action potential minimovies demonstrate topics ranging from increasing the stimulus amplitude to the effect of cooling. In NIA2, each voltage trace in the first group is also converted to frequency for an accompanying sound track.
Beyond making lectures more engaging, the use of such movies dispels the inaccurate notions of the propagation of neuronal signals that seem to be difficult to eliminate from textbook material. For example, when students see that the action potential actually spreads out in myelinated nerve, its peak covering many nodes at once, they are usually at first bewildered, for did the text not say that it "jumped" from node to node? Soon the student has the correct notion of the breadth of the voltage change in centimeters as it propagates actively along an axon.
One particularly popular minimovie with our colleagues, derived from the Partial Demyelination tutorial, compares an action potential in unmyelinated and myelinated axon by showing the action potential as it travels along a bare length of axon and then invades a myelinated portion (Fig 4).
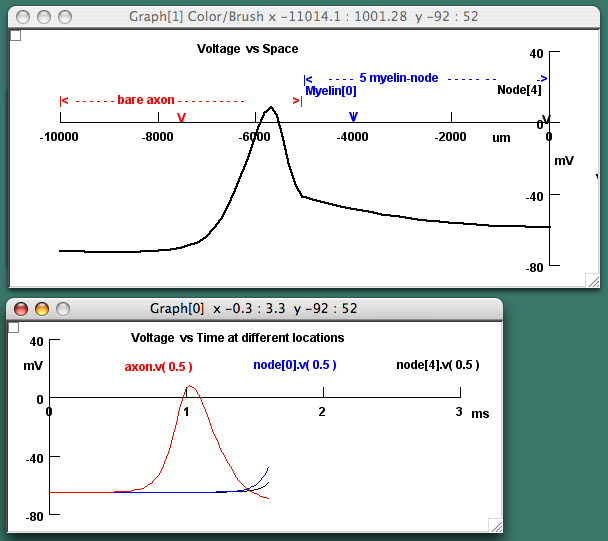
Figure 4. Arrested minimovie of the propagation of an action potential in a partially demyelinated nerve of 10 μm diameter. The upper, voltage-versus-space graph diagrams the axon as bare on the left half, with a red recording electrode in the center of the bare portion, and myelinated on the right half (5 nodes and 5 internodes each 1000 μm long), with a blue recording electrode in the first node and a black recording electrode in the last node. The movie was stopped when the peak of the action potential, triggered at the left end, was just about to invade the myelinated half, the depolarization spreading far in front of the impulse. The lower, voltage-versus-time graph shows the complete action potential, since it has passed the red electrode, and the arrested depolarization at the blue and black electrodes.
At first, students find it almost shocking to see the change in the action potential from a rather narrow event in space, its entire overshoot extending over less than 0.5 mm in the bare axon, to a voltage change that remains at peak amplitude over the whole 5 mm of myelinated axon in the simulation! As the action potential traveling in the bare axon approaches the myelinated portion, the depolarization spreading far in front of itself makes absolutely clear how propagation occurs. A separate minimovie of only myelinated nerve shows a stretch that is long enough so the student can see the tiny bumps and dips on the voltage trace at the nodes as the ionic currents surge in on the rising phase and out on the falling phase (Fig 5). Such detail captivates students, in our experience, surprising them and helping them to form a correct picture of what happens to the currents and the voltage at the nodes and internodes. A voltage-versus-time graph associated with this movie that plots the action potential as it passes two locations in the axon is useful in showing medical students, in particular, how conduction velocity is calculated, an essential technique in neurology.
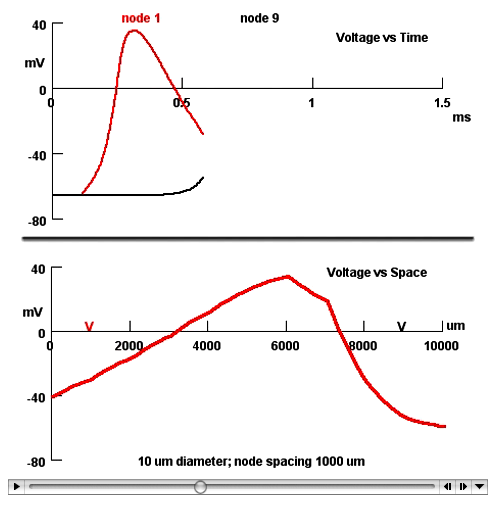
Figure 5. Arrested minimovie of an action potential in a myelinated nerve recorded by electrodes at node 1 and node 9 of 10 nodes 1000 μm apart. The movie has been stopped when the peak of the action potential is at the 6000 μm node. The upper, voltage-versus-time graph shows a complete trace recorded by the red electrode at node 1 and the beginning of the depolarization as the impulse approaches the black electrode at node 9. The lower, voltage-versus-space graph shows the electrode positions. The action potential was started at the left end of the axon.
Students often become engaged when NIA simulations are used interactively at the podium. Running simulations during the lecture is relatively easy, since toggling between PowerPoint slides and a simulation is straightforward on either a PC or a Mac. The effective strategy is to ask the students, for example, "What do you expect will happen to the peak of this action potential if I increase the external sodium concentration? How many of you vote that it will get bigger? How many vote for smaller? How many aren't sure?" A little practice might be necessary before the lecture, but even in a class of over one hundred students this method can work, providing a degree of suspense that is uncommon in standard lectures and causing even the tuned-out students to be curious about the outcome.
A popular NIA tutorial to use in this fashion is, again, Partial Demyelination, but this time stimulated at the myelinated end. When the action potential is triggered at the far right end of the myelinated portion, it propagates normally along the myelinated half but then falters at the junction between the myelinated and demyelinated halves. Hesitating, it ultimately fails to invade the bare axon. Since this condition mimics that of multiple sclerosis, it never fails to intrigue students.
The instructor might now ask, "What bare axon parameters can we change to make this action potential invade and propagate in the bare axon? If we were to set out to look for a drug to help this multiple sclerosis patient, what would we want our drug to do?" Ultimately the class can suggest a drug that partially blocks potassium channels; one that increases the density of the sodium channels in the membrane might be suggested at first but, after discussion, the students might conclude that such a drug would be difficult to design from a biological standpoint. But either a decrease in the potassium channel density or an increase in the sodium channel density in the simulation will lead to invasion of the bare axon and reinforce the students' understanding of threshold as a tug-of-war between the sodium and potassium currents. What about a change in diameter (which tests the students' understanding of capacitance as membrane area)? What about an overdose of the potassium channel blocker, a complete block (which tests their knowledge of the mechanism of the action potential)? Would this overdose kill the patient? (Yes.)
Would a change in temperature facilitate invasion of the bare axon? Multiple sclerosis patients are known to prefer certain temperatures ( Humm et al. 2004 ), but, based on neurophysiology, can the students predict whether the patient might prefer to be cool or warm? Changing the temperature in this tutorial is among the most suspenseful and effective of all of the interactive simulations, probably because of its immediate relation to the disease and perhaps, also, because the temperature change required to permit propagation into the bare axon is so small. When the correct change is made, the action potential hesitates for an excruciatingly long time, finally becoming regenerative and even leading to student applause in our experience! The final answer seems at first counterintuitive but effectively solidifies a student's understanding that channel transitions underlie the action potential and these transitions -- from closed to open to inactivated states -- obey Michalis-Menten kinetics and temperature sensitivity.
Within NIA, essentially any tutorial can be used to engage student interest through suspense. In the Chattering Ion Channels tutorial, the single channel simulations, the lecturer can specify the type of channel in the patch and its number (Fig 6). When there is a single channel, each delivery of the depolarizing pulse leads to a different result because channel opening is stochastic. Ergo the suspense: is there an opening? Does the direction of the current tell us which channel it is? When is the opening -- early or late in the pulse? One opening, or two, or maybe more? Would so many openings be expected of this type of channel? We also find suspense when incrementally increasing the channel number in the patch from one to two, then three, then, ultimately, to hundreds, thousands, and tens of thousands to reveal how macroscopic currents are built up. The "What If? approach" in lecture leads to understanding through engaging curiosity about the outcome.
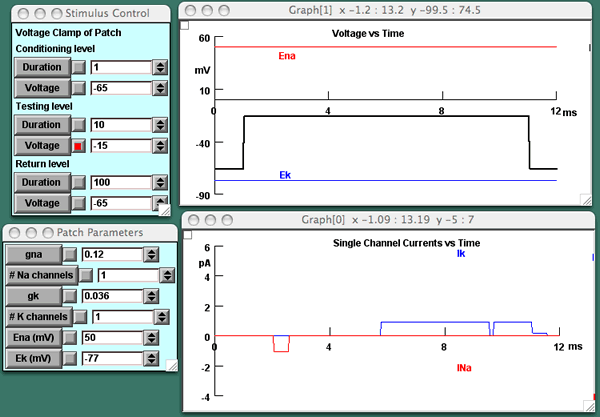
Fig 6. The results of one run of a voltage step from -65 mV to -15 mV and back (see the Stimulus control panel) in the Chattering Ion Channels tutorial. The patch in this simulation contains one Na channel and one K channel, as specified in the Patch Parameters menu. Clearly the Na channel has opened once and either closed or inactivated; the K channel has opened and closed twice, the second closing happening after the voltage step is over, leading to a smaller outward current through the channel after the step.
Turning students loose with NIA in a computer lab
If NIA has been introduced in lecture and used interactively, it is relatively easy to make the transition to asking the students to do whole or abbreviated tutorials on their own or in groups in a computer lab. When the time is short, we and our colleagues have found it useful to bypass the tutorials and instead hand out a limited exercise with a set of steps to follow and questions to answer at each step (and then hand in). The instructor circulates through the groups to solve any problems with the running of the simulations, to ask questions to make sure the students are understanding what they are seeing, or to ask what other parameter changes they might make in order to test the hypothesis. "So what experiment could you do here to test if your explanation is correct?" the instructor might say, holding back on giving away answers.
A number of colleagues use NIA in undergraduate neuroscience courses. One colleague used NIA1.5 for two weeks in the laboratory portion of an upper level introductory neuroscience course at Bowdoin College in Maine, USA. Each student had access to NIA1.5 in a computer lab. In the first week, the instructor led the students through several tutorials that were chosen to complement what they learned in lecture. These sessions also gave the students a chance to become facile with the program. In an innovative twist during the second week, the students, working in groups of two, designed their own hypotheses to test. The faculty member and a laboratory instructor met with each group to decide which tutorial provided the ideal parameters to manipulate and therefore was the most appropriate for running their own experiment. One hypothesis, for example, was that unmyelinated axons would be affected at lower concentrations of tetrodotoxin than myelinated axons; the students decreased the Na channel density in equal proportions in the two axon types and measured action potential rate of rise, amplitude, and conduction velocity to come to their conclusions.
Colleagues who have undergraduates do the NIA tutorials in a computer lab setting usually have made individualized materials to complement the tutorials: for example, handouts with specific exercises to be done in each tutorial (separate from those in the published tutorials), questions about the results of simulations, problem sets, and homework. Colleagues have sent us such materials for NIA1 for posting on the NIA website ( http://neuronsinaction.com ). With the publication of NIA2 and the increasing familiarity of faculty with forums on websites, this site provides a potential mechanism for sharing novel ideas about how to use these simulations and tutorials in teaching at any level.
An example of a course using NIA in conjunction with a wet lab is a course in "Principles of Neurophysiology" taught through the Department of Neurobiology and Behavior at Cornell University (Ithaca, NY, USA). The purpose of this upper level, undergraduate course is to introduce students to the power of neural computation as a tool in the laboratory as well as providing a sophisticated laboratory experience in neurophysiology. To this end, the NIA tutorials are integrated with wet lab experiments designed primarily around the Crawdad software package. Although in previous years a textbook had been used for this course, NIA is now the only text; the students refresh and deepen their understanding of neurophysiological concepts through NIA's hyperlinked materials.
The students begin with an RC circuit in the lab and the Membrane Tutorial from NIA. From then on, the tutorials are selected to complement the wet lab preparations rather than being followed in their own order. For example, the next wet lab technique to be learned is extracellular recording; here the Unmyelinated Axon and/or Myelinated Axon tutorials from NIA are useful where conduction velocity can be measured by inserting two virtual electrodes into an axon. As the wet lab moves on to intracellular recording from crayfish muscle fibers and then snail neurons, the NIA tutorials dealing with equilibrium and resting potentials, action potentials and threshold, excitatory and inhibitory synaptic potentials, and then voltage clamp, are introduced as appropriate.
For the midterm and final "exams" in this course, the students must execute a project of their own design that blends the wet and virtual lab experiences and then write it up. In one example of such a project, students recorded the extracellular action potentials from all six axons in the third motor nerve of the crayfish, calculated the six conduction velocities from these recordings, and then estimated their diameter through simulations using NIA. In another project, students recorded action potentials and ionic currents from snail neuronal somata, added potassium channel blockers at increasing concentrations, recorded the changes in amplitude and duration of the action potential and amplitude of the potassium currents, and then simulated these results with the Action Potential and Voltage Clamp tutorials. The students had to design the projects themselves, including figuring out which simulations were appropriate. The instructor finds these "exams" to be an especially rewarding aspect of the course as students truly understand the value of using simulations to illuminate real experiments.
At the graduate level, there is typically more time to go into depth with the NIA tutorials. Intrigued neuroscience students can venture into the Advanced tutorials. We also know of colleagues who have assigned NIA to physics students, biomedical engineering students, and computer science students interested in neurons; as well, some colleagues who use simulations in their research have told us that they assign NIA to students joining their labs as an introduction to computational neuroscience and to NEURON.
One of the authors and her immediate colleagues have used NIA for several years in four very different graduate student courses offered through the Cell and Molecular Physiology Department or the Neurobiology Curriculum at the University of North Carolina at Chapel Hill, detailed below. In each case, the tutorials are primarily done by individual students or groups of students working together rather than by the faculty member.
(1) The individual student
An individual student did the entire set of tutorials as a one-person course, meeting with the author once a week to clear up questions, to be "tested" on what he had learned, and to suggest experiments he had designed beyond those outlined in the tutorial. NIA was originally designed to be used in this way by the individual graduate student who might wish to test his or her knowledge of neurophysiology and to have a different learning experience from that provided by textbooks. Certainly this approach can lead to an in-depth use of the software.
(2) An introductory physiology course
In the Cell and Molecular Physiology Department, first-year graduate students taking an introductory course in human physiology use NIA each year as their introduction not only to neurons but to concepts like membrane potentials, equilibrium potentials, and driving force, concepts that apply to all cells of the body. The students are typically assigned problem sets that they are expected to solve after becoming familiar with particular tutorials. Such problem sets (and exam problems) are easily constructed by the instructor using graphs and panels captured from an NIA simulation; the student must explain what is being illustrated by the traces.
As an example of a problem, the student is given voltage-vs-time and current-vs-time graphs from a voltage clamp experiment in the Voltage Clamping a Patch tutorial. They see a family of 17 Na currents where the voltage was stepped to the reversal potential for Na ion for a long enough time to inactivate the Na channels (5 ms), returned to the resting level (-65 mV) for increasing lengths of time (for a total of 17 traces), then stepped again to zero mV to open any available Na channels. The student must figure out that this experiment tests the time course of recovery of the Na channel population from inactivation.
For a slightly more advanced problem, the students are asked to calculate the first order rate constant that applies to this problem and then to calculate it as a function of voltage by choosing different voltages for the recovery step. The problem can also be put in reverse: The instructor can give the students the answer and ask them to come up with the simulation, as in "Using NIA, demonstrate the time course of recovery from inactivation and compare the time courses at three different voltages."
(3) A course in biophysics and ion channels
NIA is used in a 6-week block devoted to biophysics and ion channels within a year-long Cellular and Molecular Neurobiology course in the Neurobiology Curriculum. The students do individual tutorials in groups of 4 outside of class and then present them as a group to the rest of the class. Since this class is also focused on reading original papers, it is particularly useful when a tutorial such as the Chattering Ion Channels tutorial (NIA2) can be paired with papers describing single channel behavior. As another example, the simulations in the Site of Impulse Initiation and Synaptic Integration tutorials are useful in discussing the current literature on this problem ( Palmer and Stuart 2006 ; Stuart and Palmer 2006 ). In this vein, the tutorial on Impulse Invasion of the Presynaptic Terminal is one that arises from a paper published by one of the authors, in which simulations were used to interpret experimental data. The inclusion of this tutorial in NIA has the double purpose of educating the student first about the presynaptic terminal and, second, about the use of computational approaches in research.
(4) 18 hours of NIA in a week!
NIA is the core tool for introducing basic neuronal function to graduate students each summer in a course at the Marine Biological Laboratory (Woods Hole, MA, USA; the Summer Program in Neuroscience, Ethics, and Behavior). The students in this course come from a variety of backgrounds -- from physics to neuroscience to psychology. They used NIA1, and now NIA2, in groups every morning (3 hours) for 6 sequential days, everyone doing the same Basic Patch tutorials at the beginning of the week after an introduction by the instructor but then specializing in different tutorials as the week progresses. The students present their particular tutorial to the other students after they have mastered it; typically they also master the hypothesis-testing style of the tutorials in their presentations. For example, they ask their peers if they can reason out what they think will happen before running a simulation. The students take on tutorials appropriate to their backgrounds; those who come to the course trained in more quantitative areas, such as math and physics, tend to take on tutorials from the Advanced section such as A Dynamical View of Threshold or Na and K Channel Kinetics and explain them to their colleagues.
Throughout NIA, we have accompanied the simulations with translational examples that colleagues might use in capturing the interest of premed students or in teaching medical students. The Partial Demyelination tutorial, detailed above, gives the lecturer the chance to explain many basic properties of neurons in the context of multiple sclerosis, from the "war" between the Na and the K currents that constitutes threshold to the forward spread of the depolarization that enables propagation to the refractory period that prevents the action potential from propagating backwards. At a more sophisticated level, the Na and K Channel Kinetics tutorial in the Advanced Patch section (Version 2) relates to chronic pain and other issues of neuronal hyperexcitability. Recent research has revealed that a particular Na channel subtype is upregulated in damaged neurons, leading to increased excitability; this tutorial explores how different channel subtypes, with differing kinetics, can determine the excitability of neurons (Fig 7). As well, the tutorial shows how certain rather well-known toxins, such as brevetoxin from one type of red tide or maculotoxin from the blue-ringed octopus, can disrupt normal neuronal function not by blocking the channel but by altering its kinetics.
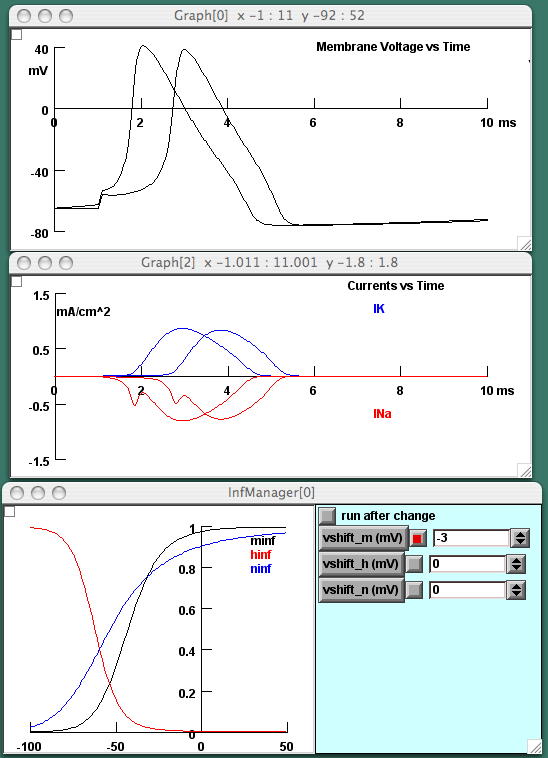
Fig 7. Panels from the Na and K Channel Kinetics tutorial. In the lowermost panel, the user can shift the steady-state values of the Hodgkin-Huxley parameters that describe the activation "m" and inactivation "h" of the Na channel and the activation "n" of the K channel. The uppermost panel shows the effect of this shift on the excitability of the neuron; in this case, a negative shift of 3 mV in the "m" curve causes the cell to become more excitable so that the action potential arises earlier in the depolarizing pulse than it did when "m" was set to zero. The middle graph shows the Na (red) and K (blue) currents during each action potential.
Other translational examples in NIA2 include:
-
In the Equilibrium Potentials tutorial, a discussion of the clinical consequences of changing extracellular ion concentrations;
-
In the Na Action Potential tutorial, a comparison of the effects on the action potential of anesthetics that block both the Na and the K channel with poisons like tetrodotoxin that block only one channel type;
-
In the Threshold: To Fire or Not To Fire tutorial, the neurophysiological basis of treating myasthenia gravis and its relation to threshold;
-
In The Ca Action Potential tutorial, the generation of a cardiac-like action potential;
-
In the Postsynaptic Inhibition tutorial, an understanding of "The GABAergic Synapse, Target of Psychoactive Drugs;"
-
In the Extracellular Ca Sensitivity of the Na Channel tutorial, how abnormal serum Ca concentration might lead to clinical problems through its effects on the voltage-gated Na channel;
-
In the Non-uniform Channel Density tutorial, propagation of an action potential through a region of axon that is partially blocked with an anesthetic.
These various clinical examples can be used to spice up a lecture on basic neuronal properties. In one instance, a practicing neurologist became excited by the clinical implications of the Partial Demyelination simulations and told us of the Uhthoff phenomenon ( Uhthoff, 1890 ) that describes the temperature sensitivity of multiple sclerosis patients. Thus the medical student who will go on in neurology might be attracted to pursue basic neurophysiology in greater detail through the NIA tutorials.
We have illustrated how Neurons In Action is used "in action" in a variety of teaching situations: in didactic and interactive lectures to undergraduates or medical students; in undergraduate laboratories, either dry or in conjunction with a wet lab; in graduate courses where the simulations can be explored extensively; or as a one-person course with an individual student. In doing so, we hope to have offered a new idea or two to colleagues using interactive software, whether NIA or another tool, to educate students of differing backgrounds in the possibly intimidating, certainly intricate, subject of neurophysiology. We are convinced that real understanding comes from the immediate feedback students receive when they can formulate and test questions arising from their progressive understanding. Such feedback is obtained readily from virtual experimentation but not so easily from textbooks or lectures.
Neurons in Action Version 2 was supported by Course, Curriculum, and Laboratory Improvement grant #0442748 from the Department of Undergraduate Education of the National Science Foundation, USA.
Carnevale NT, Hines ML (2006). The NEURON Book. Cambridge Univ Press, Cambridge UK.
Hines ML, Carnevale NT (1997). The NEURON simulation environment. Neural Computation 9:1179-1209.
Hines ML, Carnevale NT (2001). NEURON: a tool for neuroscientists. Neuroscientist 7:123-35. http://www.neuron.yale.edu
Humm AM, Beer S, Kool J, Magistris MR, Kesselring J, Rosler KM (2004). Quantification of Uhthoff's phenomenon in multiple sclerosis: a magnetic stimulation study. Clin Neurophysiol. 115:2493-501.
Moore JW, Stuart AE (2000). Neurons in Action: Computer Simulations with NeuroLab. Sinauer Associates, Sunderland, MA, USA.
Moore JW, Stuart AE (2004). Neurons in Action: Computer Simulations with NeuroLab. Version 1.5. Sinauer Associates, Sunderland, MA, USA.
Moore JW, Stuart AE (2007). Neurons in Action Version 2: Tutorials and Simulations Using NEURON. Sinauer Associates, Sunderland, MA, USA. http://neuronsinaction.com
Palmer LM, Stuart GJ (2006). Action potential initiation in layer 5 pyramidal neurons. J. Neurosci 26, 1854-1863.
Stuart GJ, Palmer LM (2006). Imaging membrane potential in dendrites and axons of single neurons. Pfleugers Arch 453, 403-410.
Uhthoff W (1890). Untersuchungen über die bei der multiplen Herdsklerose vorkommenden Augenstörungen. Arch. Psychiat. Nervenkr. 21, pp. 55-116; 303-410.
License
Any party may pass on this Work by electronic means and make it available for download under the terms and conditions of the Digital Peer Publishing License. The text of the license may be accessed and retrieved via Internet at http://www.dipp.nrw.de/lizenzen/dppl/dppl/DPPL_v2_en_06-2004.html
